Abstract
Background:NPM1 is mutated in approximately 30% of patients with acute myeloid leukemia (AML) and is one of the mutations that defines favorable risk by European LeukemiaNet (ELN) 2017 criteria. Mutations that are highly specific for secondary AML including ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, and ZRSR2 (sMut) (Lindsley et al.) have been shown to confer poor prognosis. The overall impact of these mutations in the setting of NPM1-mutated AML remains unclear.
Objective: In this study, we examined the outcomes in patients with NPM1-mutated AML with other potential adverse prognostic factors including sMut and measurable residual disease (MRD).
Methods: This was a multicenter, retrospective study (Moffitt Cancer Center, Weill Cornell, and Memorial Healthcare System) of NPM1-mutated AML patients who were diagnosed and treated from 2013 to June 2022. Inclusion was restricted to patients with NPM1-mutated AML who had mutation analysis (NGS) performed at diagnosis (n=233). Kaplan-Meier, univariate (log-rank), and multivariate (Cox regression) analyses were performed.
Results: Among 233 patients (111M/122F, median age 63 (22-86) at diagnosis), 81.1% had de novo AML (dAML). By ELN 2017 criteria, 58.8% (137/233) had favorable risk, 29.2% (68/233) had intermediate risk, and 9.4% (22/233) had adverse risk disease. Over 88% had intermediate risk cytogenetics at the time of diagnosis. Common co-mutations included DNMT3A (48.9%), FLT3-ITD (33.9%), TET2 (28.7%), IDH1 (15.8%), FLT3-TKD (15%), NRAS (12.4%), and IDH2 (11.6%) (Table 1).
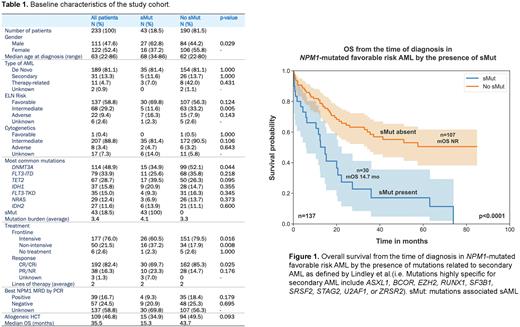
Forty-three (18.5%) patients had sMut, 30 (69.8%) of whom had ELN favorable risk disease (median age 67, range 46-86). Patients with sMut achieved a CR/CRi rate of 69.7% (30/43) compared to 85.3% (162/190) of patients without sMut (p=0.025). Approximately 35% of the sMut cohort proceeded to transplant compared to 50% in those without sMut (p=0.093). The median overall survival (mOS) of the entire cohort was 35.5 months with a median follow up of 52.1 months. Those who harbored sMut had worse OS compared to those without sMut (15.3 months vs 43.7 months, p=0.002). Among patients with ELN favorable-risk disease, OS was 14.7 months vs. not reached for those with sMut and without sMut, respectively (p<0.0001) (Figure 1).
For patients with ELN favorable risk, univariate analysis showed age over 60 at diagnosis, therapy-related AML (tAML), and sMut significantly impacted OS (age over 60: HR 2.67, 95% CI: 1.49-4.76, p<0.001; tAML (vs. dAML): HR 4.34, 95% CI: 1.54-12.27, p=0.006; sMut: HR 2.95, 95% CI: 1.76-4.95, p<0.001). Multivariate analysis using covariates including age over 60, AML type, sMut, and mutation burden (3 or more) confirmed the prognostic significance of sMut on OS (sMut: HR 2.12, 95% CI: 1.16-3.87, p=0.015).
Post-treatment MRD testing by qPCR for mutant NPM1 was performed in 96/233 (41.2%) patients. Patients who achieved MRD negativity predicted for longer OS compared to those with MRD positivity (MRD negative: mOS 73.9 months vs. MRD positive: mOS 34.5 months, p=0.009). Similarly, MRD negativity predicted for longer OS in the sMut subset (n=13 [9 MRD negative and 4 MRD positive], mOS 73.9 vs. 12.3 months, p=0.017). Further subgroup analysis using the favorable risk sMut cohort again showed statistical significance based on MRD status (n=8 [6 MRD negative and 2 MRD positive], mOS 27.3 vs. 10.5 months, p=0.009).
Conclusions: Our findings demonstrate that the co-occurrence sMut confers a significantly adverse effect on the survival of NPM1-mutated AML, despite being primarily classified as favorable risk by ELN 2017. These data support the recent ELN 2022 recommendations of sMut as an adverse prognostic indicator, even in the setting of otherwise favorable risk categorization. MRD negativity for NPM1 mutations was associated with improved OS, even in the presence of sMut, suggesting its importance as a prognostic factor across different risk groups.
Disclosures
Chan:Syntrix Pharmaceuticals: Research Funding. Hussaini:Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Decibio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Diaceutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Guidepoint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Tegus: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Padron:Blueprint: Honoraria; Kura: Research Funding; Incyte: Research Funding; BMS: Research Funding; Syntrix Pharmaceuticals: Research Funding; Taiho: Honoraria; Stemline: Honoraria. Roboz:Amgen: Consultancy; Celltrion: Consultancy, Other: Travel and accommodation expenses; Actinium: Consultancy; Pfizer: Consultancy, Honoraria, Other: Travel and accommodation expenses; Mesoblast: Consultancy; Helsinn Therapeutics: Consultancy; Roche: Consultancy; Bristol Myers Squibb: Consultancy; Otsuka: Consultancy; MEI Pharma: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Sandoz: Consultancy, Other: Travel and accommodation expenses; Bayer: Consultancy, Other: Travel and accommodation expenses; Amphivena Therapeutics: Other: Travel and accommodation expenses, Research Funding; Array BioPharma: Other: Travel and accommodation expenses; Clovis Oncology: Other: Travel and accommodation expenses; Sunesis Pharmaceuticals: Other: Travel and accommodation expenses, Research Funding; Eisai: Other: Travel and accommodation expenses; CTI: Research Funding; Karyopharm Therapeutics: Research Funding; Jasper Therapeutics: Consultancy; Jazz: Consultancy, Other: travel; Takeda: Consultancy; Astex Pharmaceuticals: Consultancy, Other: Travel and Accommodation expenses, Research Funding; MedImmune: Consultancy, Research Funding; Astellas: Consultancy; Genentech/Roche: Consultancy, Other: Travel and accommodation expenses; Agios: Consultancy, Research Funding; AbbVie: Consultancy, Other: travel and accommodations, Research Funding; Novartis: Consultancy, Other: Travel and accommodation expenses, Research Funding; Janssen: Consultancy, Other: travel and accommodation expenses, Research Funding; GlaxoSmithKline: Consultancy; Celgene: Consultancy, Other: travel and accommodation expenses, Research Funding; Bristol Myers Squibb: Consultancy; Mofitt Cancer Center: Research Funding; Amgen: Consultancy, Other: travel; Agios: Other: travel, Research Funding; Onconova Therapeutics: Research Funding; Tensha Therapeutics: Research Funding. Desai:Janssen Research: Research Funding; Takeda, Bristol Myers Squibb, Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sallman:BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Syndax: Membership on an entity's Board of Directors or advisory committees; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Nemucore: Membership on an entity's Board of Directors or advisory committees; Lixte: Patents & Royalties: LB-100; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Syntrix Pharmaceuticals: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; Intellia: Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Agios: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Kite: Membership on an entity's Board of Directors or advisory committees. Sweet:Syntrix Pharmaceuticals: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Komrokji:Geron: Consultancy; Servier: Consultancy, Honoraria, Speakers Bureau; CTI BioPharma, Innovent: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Speakers Bureau; Acceleron Pharma: Consultancy; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Lancet:Syntrix Pharmaceuticals: Research Funding; Astellas: Consultancy; Agios/Servio: Consultancy; Dava Oncology: Consultancy; Boxer Capital: Consultancy; Dedham Group: Consultancy; Jasper Therapeutics: Consultancy; Novartis: Consultancy; Jazz: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy; Servier: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal